Molecular Microbiology (Virology)
Institute of Medical Microbiology and Hygiene
University Regensburg
HIV, the etiological agent of acquired immunodeficiency syndrome (AIDS), remains a prominent infectious disease, with over 80 million infections since its emergence in the mid-20th century. Approximately 38 million individuals were living with HIV in 2021 (blue), while around 40 million have succumbed to AIDS since the pandemic’s onset (grey). Prevention initiatives have contributed to declining annual infection rates (red), while potent antiretroviral therapies have reduced mortality (black), currently benefiting approximately 30 million individuals (green). HIV’s global prevalence is notable, with Sub-Saharan Africa being particularly affected.
Despite extensive efforts over four decades, developing an effective prophylactic vaccine remains a challenge due to HIV’s extensive variability and its ability to evade immune responses. Overcoming these hurdles is crucial to effectively combatting the HIV pandemic.
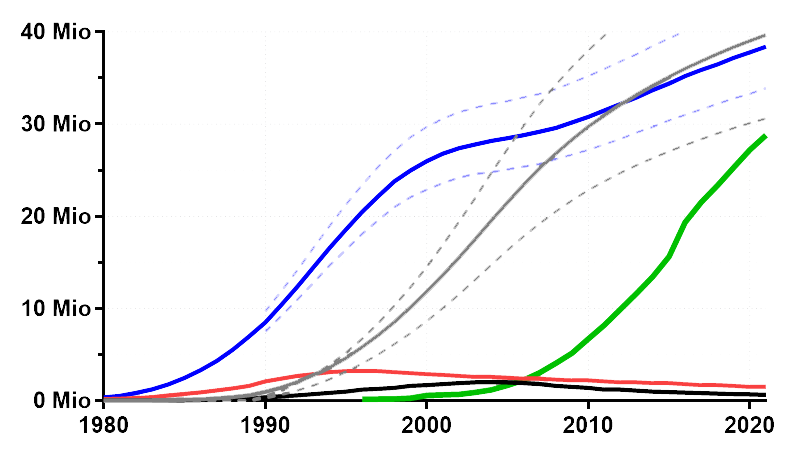
Global development of the HIV pandemic (# of individuals) from the early 80ies until 2022
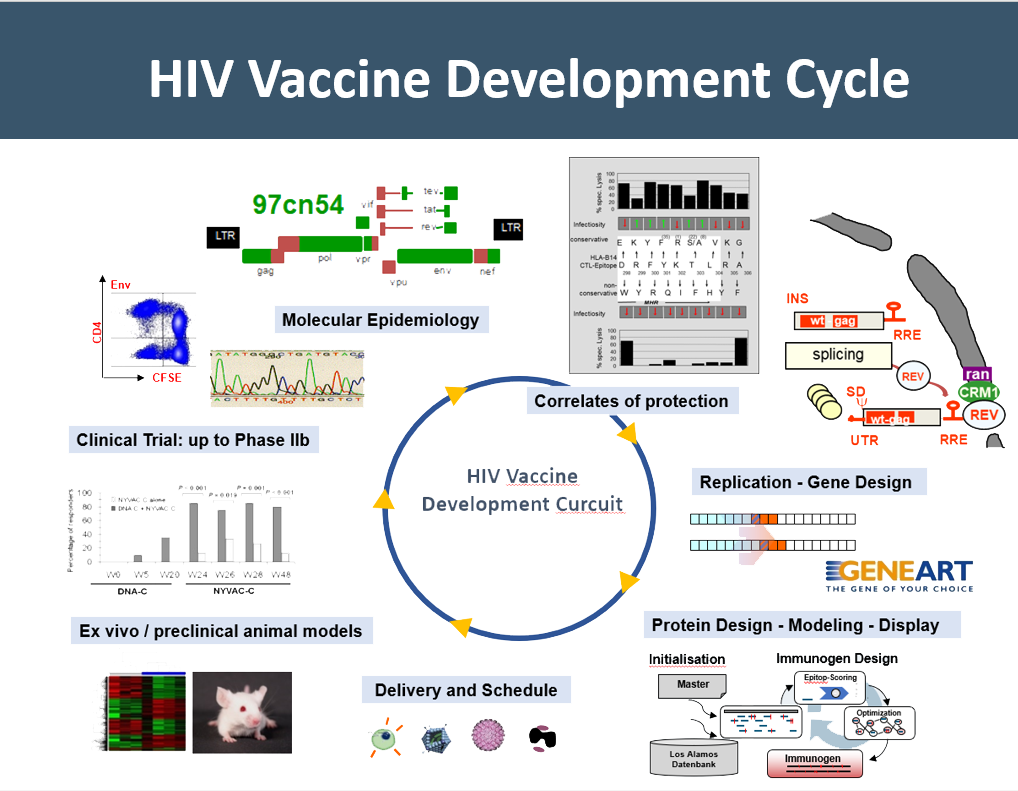
Our group is dedicated to HIV vaccine development for more than 30 years. In iterative cycles, comprising a pleiotropy of technologies such as molecular epidemiology, gene and protein design, various antigen delivery modalities, preclinical testing, and clinical validation, we have developed three generations of HIV vaccines. This work has been supported by various national, European, and global funding organizations as well as partners from academia and industry from more than 20 different countries across the years.
As part of this project, our group also pioneered RNA- and codon-optimization together with next-generation gene design and synthesis technologies to advance immunogen design and vaccine efficiency. Besides providing at that time innovative DNA vaccines to EuroVacc, this led to the foundation of GeneArt GmbH, a Germany-based biotech company, which went public in 2006 and was acquired with its more than 200 employees and two subsidiaries in North America by Life Technologies Inc. (now Thermofisher Inc.).
The first-generation is based on a representative isolate of HIV clade C (causing about half of all infections) called 97CN54 from China, with a focus on conserved T cell antigens (GagPolNef) supplemented by the external surface protein gp120. Within the EU-funded project Eurovacc, these antigens have been evaluated in animal models and phase I clinical trials.
The second-generation vaccine has been adopted within PTVDC (Poxvirus T-cell Vaccine Developing Cluster), a project that was funded by the Bill and Melinda Gates Foundation, to an African C clade isolate called 96ZM651 and modified to induce better-balanced T cell response and more potent antibody responses. Rigorous testing of these antigens in animal models has paved the way for testing in humans. Our DNA vaccine “PT123” is currently being used in the Phase 2b PrepVacc clinical efficacy trial in South Africa.
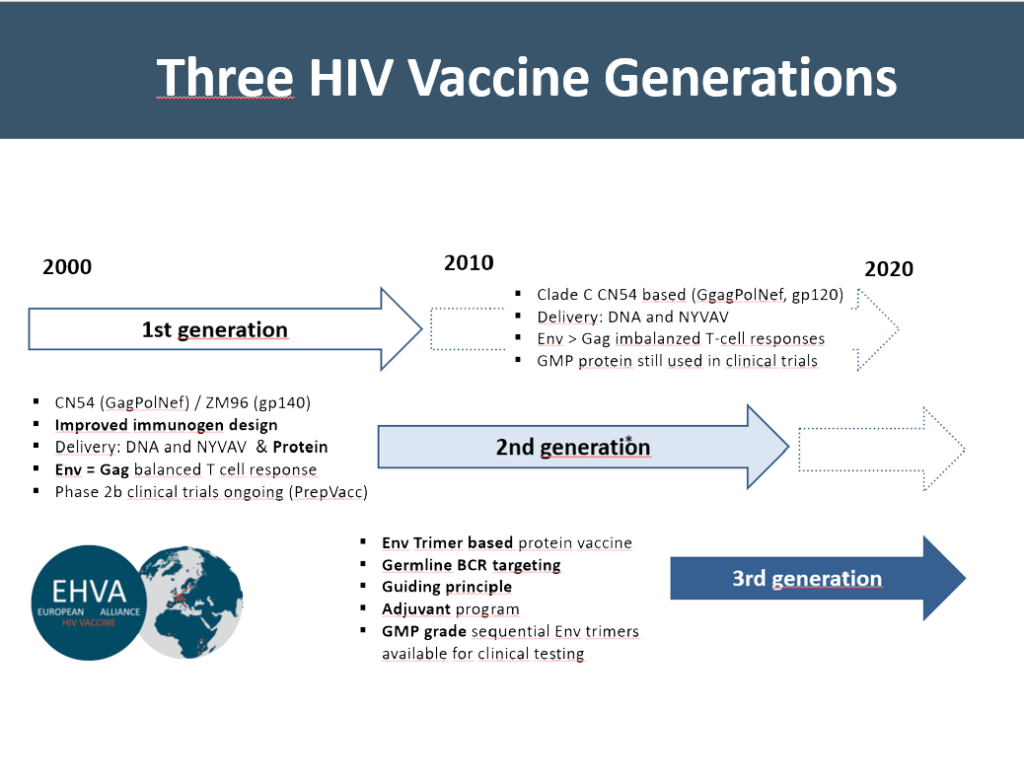
Meanwhile, within the EU H2020-funded project EHVA (European HIV Vaccine Alliance), we have generated further improved antigens that are enriched for T-cell epitopes. The main improvement in our third-generation vaccine is the development of sequential pre-fusion stabilized envelope antigens to guide B cell receptor development towards neutralization breadth. Two sequential Envelope antigens were manufactured according to Good Manufacturing Guidelines (GMP) and are now available for testing the guiding principle of B-cell development in phase 1 clinical trials. For more details, please click here: EHVA Project and EHVA Webpage.
Additionally, we are working on novel ways to deliver these pre-fusion stabilized gp140 antigens, multimerized in favourable topology on various organic and inorganic particulate carriers, directly to lymph nodes to improve germinal center reactions leading to superior antibody responses.
Human Cytomegalovirus (hCMV) is an opportunistic pathogen with global distribution and a seroprevalence ranging from 60% to 90%, depending on the geographic region. hCMV establishes a lifelong latency and is apathogenic in healthy individuals. In immunocompromised patients, e.g. solid organ and hematopoietic stem cell transplant recipients, hCMV reactivation is often associated with severe disease and transplant rejection. Transmission of hCMV to neonates during pregnancy or birth can lead to neurological damage, hearing loss, vision impairments or retardation. Several antivirals are approved but suffer from low tolerability and establishment of viral resistance. Standard diagnosis comprises serology and hCMV detection via PCR or antigen testing. Monitoring hCMV specific T cells is recommended in transplant patients to follow the reconstitution of the cell mediated and hCMV immunity.
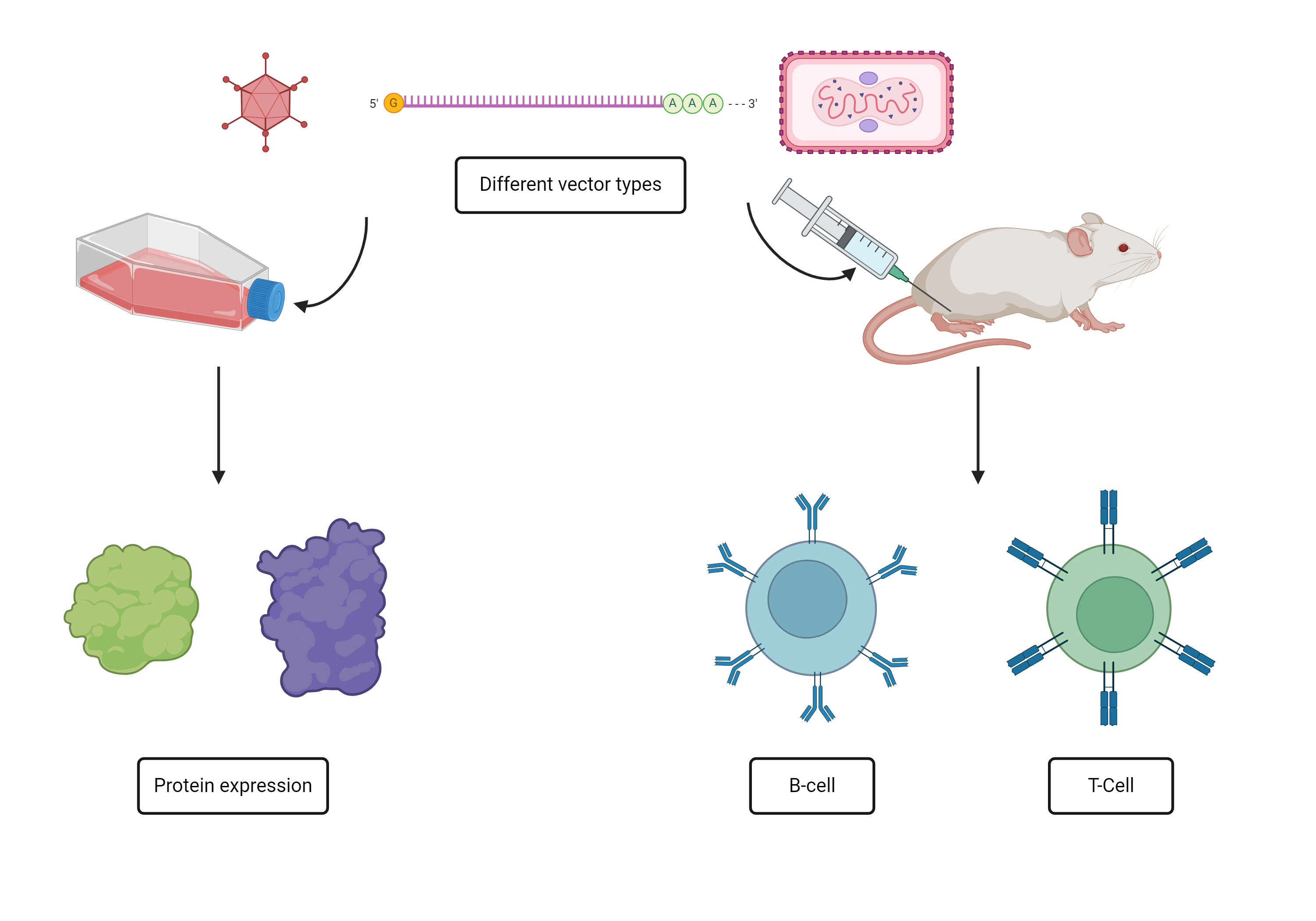
Currently, neither a prophylactic, nor therapeutic CMV vaccines are available. In collaboration with Sirion Biotech, part of Revvity, we are developing hCMV antigen payloads for delivery by several viral and mRNA based vectors as prophylactic and therapeutic vaccines. Candidates showing best humoral and cell mediated immune profiles will be recommended for future clinical development.
In another project, we focus on the development and miniaturization of a novel RTqPCR assay to revolutionize hCMV T cell monitoring. Current T cell platforms consume large blood volumes, which excludes testing of neonates and hampers regular testing of HSCT recipients. We hypothesize that the combination of antigens, biomarkers and ultrasensitive readout may allow a volume reduction to >100 µl per test.
Coronaviruses, a family of viruses known for causing respiratory illnesses in humans, have become a significant global concern in recent times. The most infamous member of this viral family, SARS-CoV-2, emerged in late 2019 as the causative agent of the coronavirus disease 19 (COVID-19) pandemic. Since its initial appearance, COVID-19 has affected billions of individuals worldwide, with millions succumbing to the severe respiratory symptoms associated with the disease. Amid the ongoing battle against COVID-19, various preventive measures, including vaccination campaigns and public health interventions, have been implemented to curb the spread of the virus. While progress has been made in managing the impact of COVID-19, the quest for a comprehensive solution, such as a highly effective prophylactic vaccine, continues. Nevertheless, the complex nature of coronaviruses, coupled with their ability to mutate and evade immune responses, poses ongoing challenges for the development of effective and universally protective vaccines.
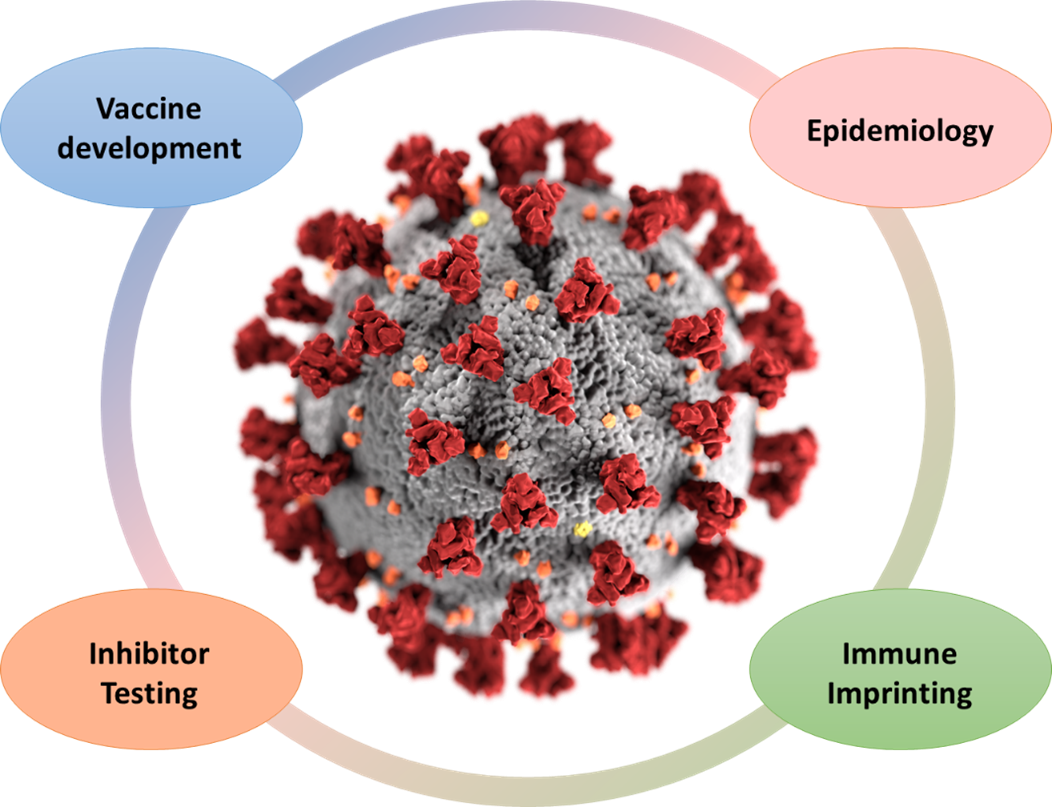
Sars-Cov-2 projects of the AG Wagner
In the initial phase of the pandemic, our team proactively engaged in the development of diagnostic platforms designed to ascertain binding and neutralizing antibodies against SARS-CoV-2. Subsequently, we addressed pivotal epidemiological questions concerning both adults and children. Our investigations encompassed seroprevalence post-initial infection waves (TiKoCo-19 study), the effectiveness of surveillance mechanisms, the discernment of symptoms, and the determination of infection fatality rates. Ongoing inquiries involve the analysis of antibody decay after vaccination and (breakthrough) infection, the assessment of immune evasion by emerging variants of concern (VOCs), and the impacts of vaccine-mediated immune imprinting related to SARS-CoV-2 VOCs (CoVako study). Together with our international collaborators, we design and test novel vaccine antigens in the pursuit of a sterile-protective pan-coronavirus vaccine. On top, we are investigating novel computationally designed small-molecule inhibitors to help fight severe disease. Our commitment remains unwavering as we contribute to the collective understanding and battle against this global health crisis.
HERVs are relics of ancient infections with exogenous retroviruses that occurred millions of years ago. These viruses were able to integrate their genome into host germ line cells. This allowed stable integration and subsequent horizontal transmission of viral DNA through Mendelian inheritance. Today, HERVs account for approximately 8% of all sequences in the human genome. Although most HERV sequences are inactive due to an accumulation of mutations and do not promote the release of infectious or non-infectious virus particles, some sequences still contain intact open reading frames. For some HERVs, these include the group-specific antigens (Gag) or the viral envelope (Env). HERV-encoded ORFs are overexpressed in certain conditions, including some cancers and certain autoimmune diseases. This makes HERV-encoded proteins attractive targets for diagnostic tumour stratification and therapeutic strategies.
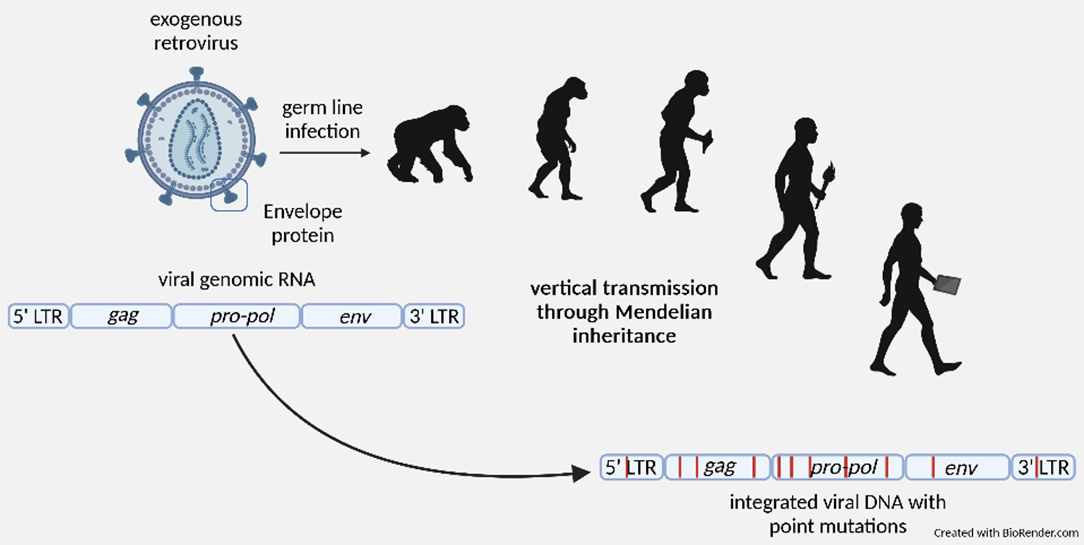
Stable integration of HERV sequences through infection of the germ line.
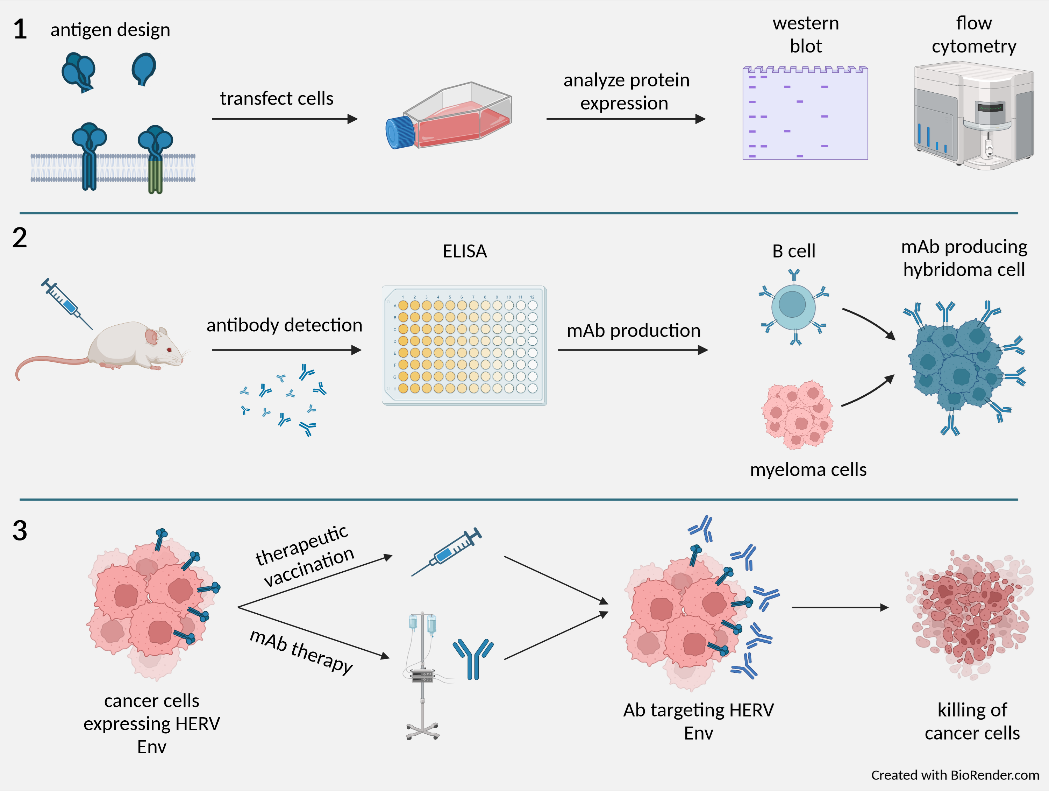
Workflow for the production, characterization and clinical application of monoclonal anti-HERV antibodies.
Our current work focuses on the Env protein of HERV-H, one of the most abundant endogenous retroviruses, as the main target for tumour stratification, companion diagnosis, and immunotherapy. Central to this project is the generation of polyclonal antisera as well as monoclonal antibodies comprising various tasks: (1) Expression, purification, and characterization of HERV-H Env derivatives; (2) immunization of New Zealand White rabbits and Balb/C mice, generation of polyclonal antisera (pAs) and monoclonal antibodies (mAbs); (3) validation of pAs and mAbs as diagnostic tool for tumour stratification; (4) humanization of mAbs, configuration as immunotoxin and in vitro testing; (4) mAb conversion into chimeric antigen receptor (CAR) for CAR-T therapy; (5) establish a mouse tumour model to support preclinical in vivo testing of the various HERV-H related immunotherapies.
This work has been initiated in a collaborative effort together with partners in Denmark and Germany (EuroStars Project; H2020).
Approximately 80% of all unvaccinated sexually active people will be infected with HPV once in their life. Despite the availability of highly efficacious prophylactic vaccines, HPV is still highly prevalent worldwide (>10%) and represents the most significant part of virus-induced tumour diseases. Infection with HPV is the leading cause of cervical dysplasia and cancer and is also associated with tumours in the other parts of the anogenital tract. HPV also causes cancers in the head and neck area, especially in the oropharynx. Thus, the global burden of HPV-related morbidity and mortality is immense (>300.000 deaths in 2020). Although prophylactic vaccines efficiently protect from virus infection, they are ineffective as a treatment for HPV-induced dysplasia or cancer. Hence, a novel therapy against HPV-induced malignancies is of the highest urgency. Natural regression of HPV-induced dysplasia is connected to rigid T-cell responses against the regulatory early proteins of the virus.
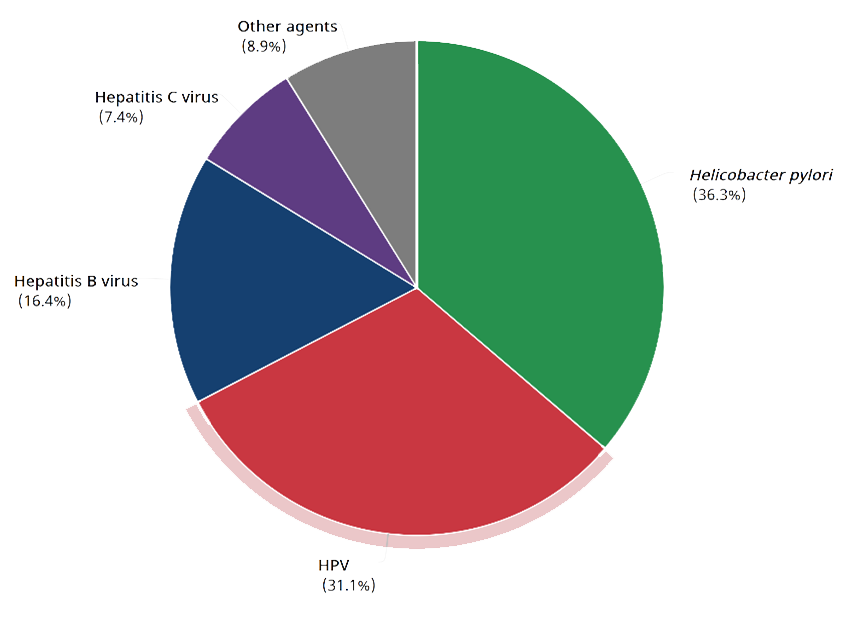
Cancer cases attributable to infectious agents in 2020 (GLOBOCAN)
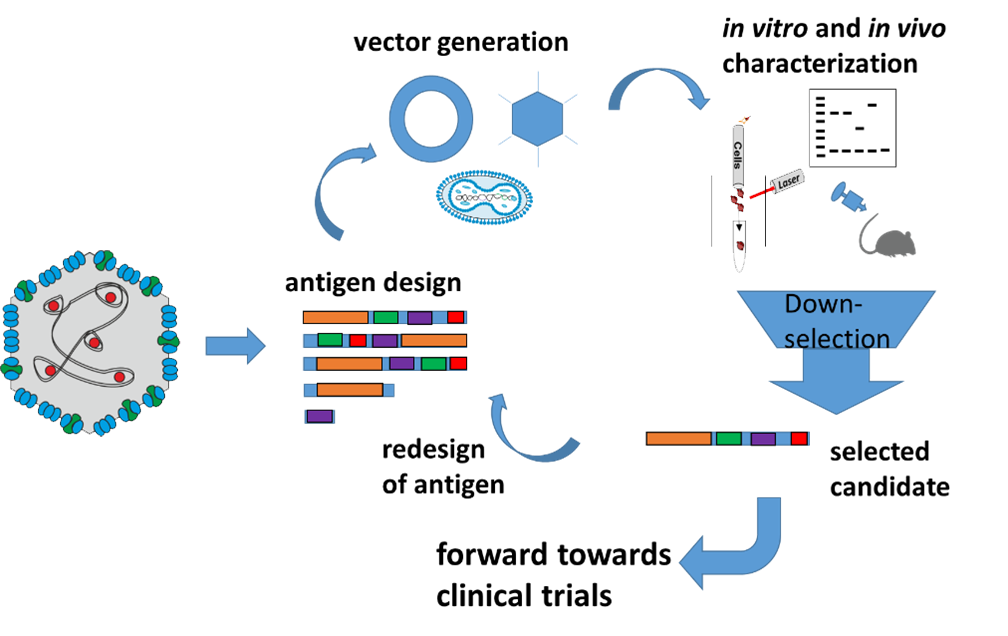
From single virus antigens towards clinical trials
To enable the treatment of HPV-derived malignancies in the early or late stages of the disease, we set out to develop a therapeutic HPV vaccine. We hypothesize that vaccine-mediated induction of cell-mediated immune mechanisms will help to eradicate pre-malignant HPV-infected cells and thus prevent progression to dysplasia and cancer.
Vaccine payloads are designed to maximize safety profiles and, at the same time, tailor immune responses to the relevant viral target moieties. Preclinical testing comprises mouse models proving protection from tumour challenge as well as non-human primate models, where the generation of cervix dysplasia and cancer, caused by a related papillomavirus shall be prevented.
Based on our encouraging preclinical data, one of the HPV16 vaccine candidates will be further developed for clinical testing together with our co-founded spin-off company LOMA Therapeutics. For further information: THERIN project
Influenza A virus (IAV), the most prevalent and diverse group of influenza viruses, continues to pose a significant public health challenge. IAVs are responsible for seasonal flu epidemics but also severe pandemics like the Spanish flu in 1918 or the more recent swine flu in 2009. IAVs are constantly evolving and thus evading the immune system: Antigenic drift accounts for minor changes in the IAV’s surface proteins, while antigenic shift enables the acquisition of entirely new surface proteins from other influenza viruses.
IAV are highly contagious and spread through respiratory droplets emitted when coughing or sneezing. To protect against influenza A viruses, there is an annually updated flu vaccine, which can reduce the risk of severe illness, hospitalization, and death. There are also some approved drugs, initially conceptualized to protect from severe illness, but so far they have only shown limited therapeutic efficacy.
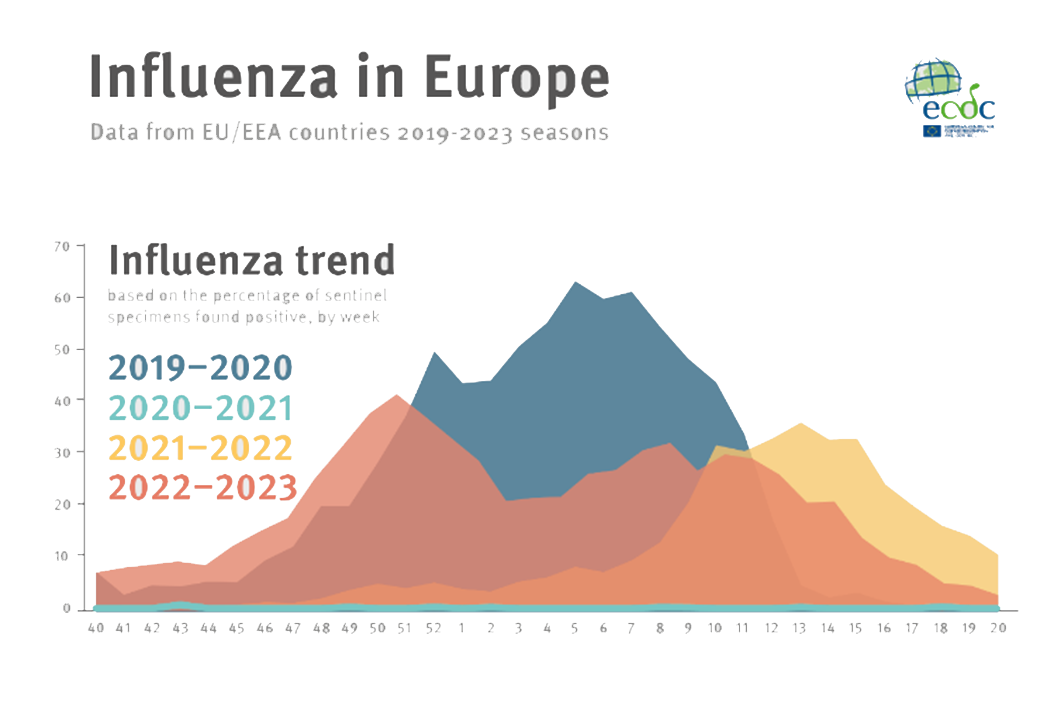
https://www.ecdc.europa.eu/sites/default/files/images/influenza-seasons-summary-2019-2023.png
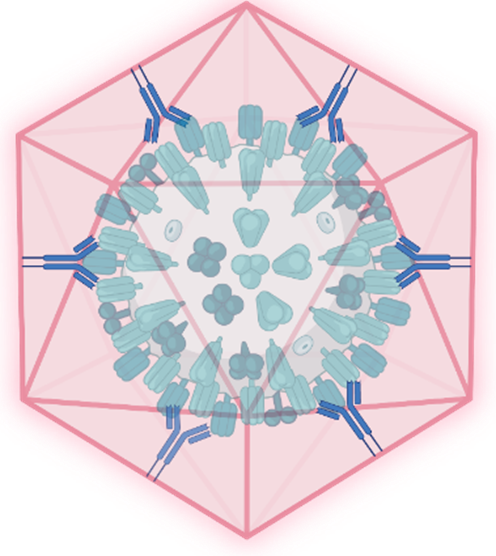
schematic of a nanoshell captured IAV
We target IAV by two complementary strategies:
Better vaccines: Since 2019, our research group has been engaged in a collaborative effort with the Heeney Lab at the University of Cambridge (UK) to develop novel influenza vaccine antigens. Our approach involves adapting codon usage to optimize the expression of antigens in mammalian cells, employing pertinent bioinformatics techniques such as consensus and ancestral computing to identify and design efficacious antigens, and evaluating diverse vehicles for vaccine delivery encompassing non-viral (DNA, mRNA) and viral vectors (adenoviral and poxviral vectors).
Novel therapeutics: Our second front in combating influenza lies within the EU-funded Virofight consortium. This project expands our anti-influenza efforts to encompass therapeutic approaches. Virofight aims to develop a versatile platform capable of capturing and neutralizing viruses, including influenza A.
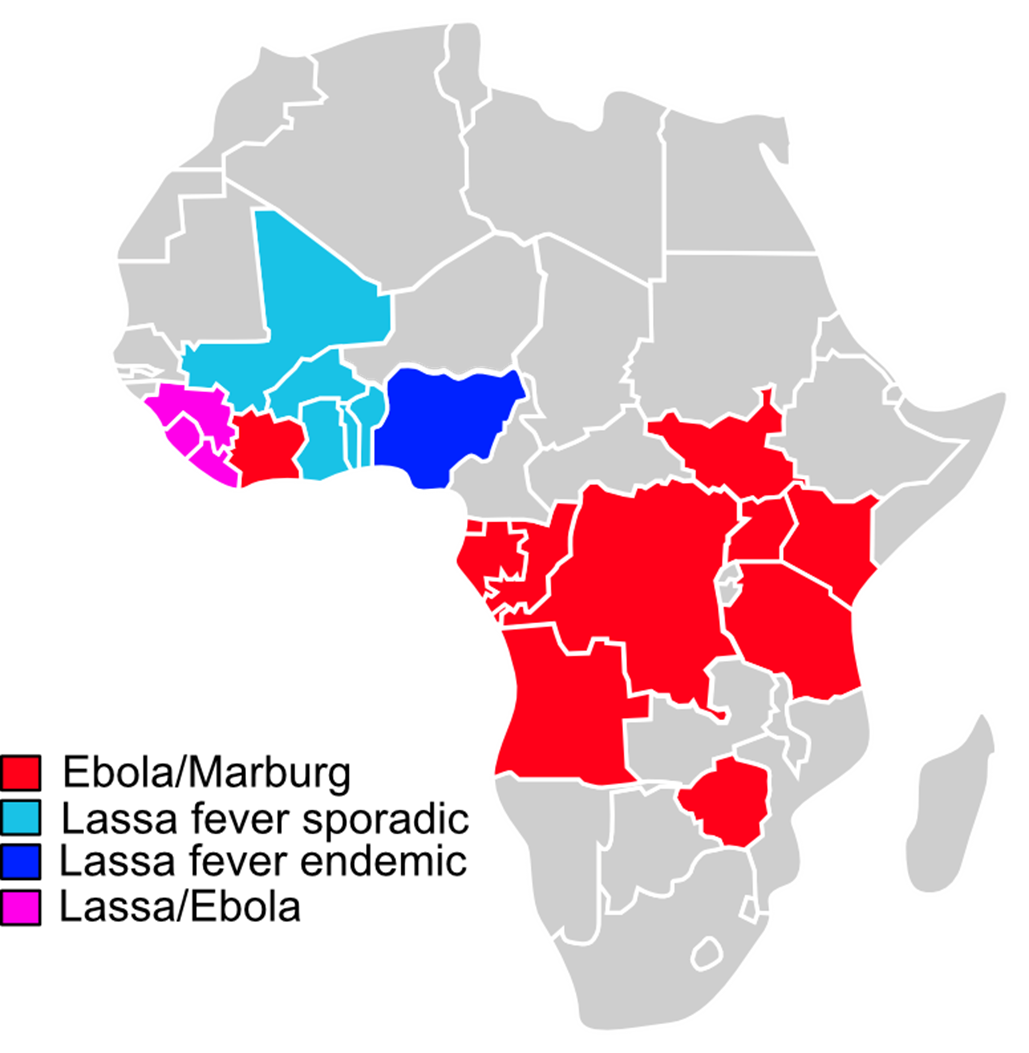
The filoviruses Ebola and Marburg virus belong to the most devastating haemorrhagic fever viruses (HFVs) in humans with a huge global epidemic potential and high case fatality rates of up to 90% depending on the virus species. Lassa fever virus is considered a dangerous pathogen too and listed as a blueprint priority disease by the WHO.
For low income developing African countries with poor health system HFVs represent one of the most serious threats to public health with a significant impact on economy. Despite the availability of vaccines against Ebola and Marburg virus disease, an effective “All in One” vaccine supporting the management of HFV outbreaks is not available yet.
Recent outbreaks of Marburg virus in several sub-Saharan Africa, the sudden outbreak of Sudan ebolavirus in Uganda and almost half a million of Lassa fever cases in West Africa every year highlight the urgent need to develop a single trivalent vaccine that protects against all three of these HFVs.
In cooperation Cambridge University (Heeney Lab, UK) and DIOSynVax (Cambridge, UK) we generated and validated the immunogenicity of DNA- and MVA-based vaccine candidates suitable for GMP manufacturing to combat EBOV, MARV and LASV haemorrhagic fever viruses. Based on immunogenicity and efficacy data in Hartley guinea pigs obtained from challenge studies against lethal doses of EBOV, MARV and LASV (PHAC, Canada) our vaccine candidate(s) will be forwarded to GMP production (ProBioGen AG, Berlin) and kick-off a phase I clinical trial in humans (3). Our next generation haemorrhagic fever virus vaccine will additionally entail antigens derived another HFV, namely Crimean Congo haemorrhagic fever virus (CCHFV), known to pose a significant threat to humans due to climate change and wide geographical distribution. Our “All in One” haemorrhagic fever vaccine candidates are planned to be used not only during outbreak scenarios but also as a prophylactic vaccine for developing regions, in which these viruses are prevalent.